by Steven Dickman, CEO, CBT Advisors
Looking for a silver lining in the current challenging climate for healthcare VC? Look no further than Domain Associates. Amid the exit drought brought on by the economic crisis, Domain has put together an impressive string of exits. By our count, Domain has had seventeen significant exits – 3x or better, sometimes much better – since 2005. Even after the doors slammed on IPOs in 2007 and the crisis hit in the fall of 2008, the exits have continued with 2009 acquisitions of BiPar (for up to $500 million to Sanofi), Calixa ($403 million to Cubist) and Corthera (up to $620 million to Novartis – see Table 1).
We hasten to add that we don’t know exactly how well Domain did in some of their investments in companies that had IPO “exits” or even if they have sold their shares. The true profitability of venture funds is known only to the funds themselves and some of their investors aka limited partners. For real accuracy, performance comparisons among venture funds should be made for funds raised in the same year (the “vintage funds” approach) and for funds with roughly the same sizes and strategies. Still, after looking at a couple of dozen top funds and were hard pressed to find any with more than five or six decent exits in the 2005-2009 time frame. Aside from Domain, The highest performers we found had seven or eight.

Therefore, Domain’s exit numbers – and, presumably, the returns that result from them – have to be the envy of the firm’s VC peers. That is especially true now, more than a year into the economic crisis. Although pharma and big biotech have been spared, the current tough times have hit VCs hard. The IPO market may come back in 2010 but don’t get your hopes up. Ironwood on February 3 was the first VC-backed biotech company to go public in 2010 and one of a handful of biotherapeutics companies to go public on any exchange since 2007. But that was not a rousing success given the “haircut” in the price ($11.25 not $14-16 per share) and the lower amount raised than planned ($187 million, not $272 million).

Worse for VCs, fund-raising is down and the returns for many if not most funds have been negative for years. The actual numbers are unknown, though Boston Biotech Watch’s parent CBT Advisors got access to some aggregate data in 2008 that was pretty sobering, showing a majority of 232 healthcare funds over twenty-two years returning barely more than their invested capital, data we will share upon request. The reasons for this fund-raising roadblock have much to do with that performance, intensified by the structural issues that have beset many of the funds’ limited partners, an issue Boston Biotech Watch covered in an earlier post.
But despite the most challenging climate in ten years or more for VC fund-raising, Domain capped off their recent run in mid-2009 with the biggest life sciences fund raised that year, the $500 million fund Domain Partners VIII, which closed in August and increased the firm’s lifetime amount raised to $2.7 billion. To the firm’s partners, accustomed from the good times to raising money in weeks, not months, the fund-raising seemed arduous – it began in January, 2009, took seven months and yielded a fund that Domain acknowledged was 28% below its original $700 million target. To the rest of the industry, of course, the fund-raising represented a rare bright spot.
Meeting some Domain partners at the JP Morgan healthcare conference last month, it occurred to us that even here, breathing the rarified air of VC triumph, one can find the roots of the current VC malaise, which is affecting the entire innovation economy. Indeed, connecting the dots through Domain’s exits yields answers to several interesting questions, to wit:
o Why, as long as it can find syndication partners, Domain ought to continue to be successful;
o Why so many other VCs will continue to struggle to the point that some will go out of business;
o And why the most urgent conversations at JP Morgan were not between biotechs and VCs, nor between bankers and VCs, but rather between VCs and big pharma.
First, the Domain success story. Let’s eliminate any doubt that Domain was “just lucky.” Yes, the number of events – in this case exits – is low, too low to consider this analysis a scientific one. But we strongly doubt that we have been fooled by randomness and that Domain’s success is a so-called Black Swan (an unpredictable outlier as described in the book of the same name by Nassim N. Taleb).
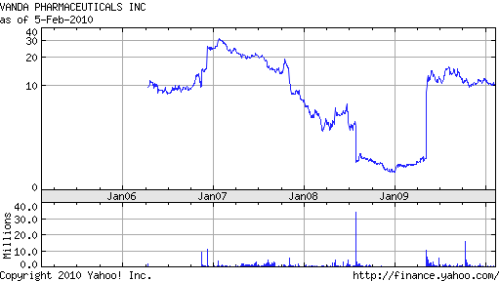
We further acknowledge that one of the presumed exits reflects the spectacular turnaround for specialty pharma shop Vanda), whose schizophrenia drug was first rejected by FDA, then approved, and is now on the market). Vanda stock was on a turbocharged roller-coaster (from $5.12 to $0.50 and all the way back up to $14.64 – all within a single year! see Figure 1) and Domain – if it sold shares – likely wound up reaping a large. But Vanda – if it was lucky, and even that is up for debate – is only one of many good exits (see Table 1).
In fact, the repeated and apparently pre-programmed success of one particular business model, layered onto an otherwise productive clinical development and medical device strategy, has made an enormous difference in the firm’s returns.
Therapies only please
Unlike some of its life sciences VC peers, Domain does not do tools or health IT or cleantech. It stays tightly focused on building companies and taking judicious clinical trial risk on promising devices or molecules – or, as in the case of Vanda and other less high-flying portfolio companies like Alimera, on promising management teams. Moving these molecules through relevant clinical milestones – especially Phase 2 trials – is a tried-and-true path to success, provided that they are the right molecules and provided the management teams execute well in moving them forward. Domain has done fine – as well as anyone in the industry – at assuming this type of clinical risk and then working hard to minimize that risk through astute management and good trial design.
But this explanation begs one question: where does Domain get the molecules? The answer: mostly from Japan. As has been well documented, Domain is particularly good at finding viable preclinical or early clinical molecules inside Japanese pharma companies, placing them in US-based companies and ushering these molecules – which are usually the most promising candidates in their Japanese originators’ pipelines – through a value inflection point, at which point they can be sold or partnered with US and European pharma companies at big premiums.

Boston Biotech Watch sat down with Eckard Weber, Domain’s resident expert at shaking loose these valuable assets from Japanese companies and shepherding them to their future homes in mostly San Diego-based companies. Weber, who until 1995 was a former university professor at UC Irvine, makes a humble and buttoned-down impression — this despite his having one of the more impressive track records in VC dealmaking the past five years, And in his initial description of how he and Domain do it, he made it sound positively pedestrian, almost trivial: “If there is unmet need with a big market opportunity, [a product] could be worth a lot of money. If you can take the product through Phase 2 for $30-40M, show safety and efficacy for that, it’s a good investment!”
So go to Japan, pick up molecules that local pharma companies have developed and license the rights for the United States and North America. Weber makes it sound so easy! But it isn’t. After all, these companies traditionally out-licensed ONLY to US and European big pharma. VCs needed not apply – until Domain’s deal with Takeda for the antibiotic Ceftaroline, out-licensing of products developed by Japanese pharma for its home market to western VC-backed companies was exceedingly rare. “What was not accepted as a business model until we came on the scene,” Weber said, “was to license the product to offshore VCs – or any VCs.”
For the first deal, Weber recounts, the major Japanese pharma took a very long time because they’d never done an out-licensing to a US VC or even a venture-backed company. That was a cultural shift that took two years. What made them eventually come around was “relationship building,” Weber said, which “is even more important in Japan than in the United States. You have to spend a great deal of time presenting your case. You need to go repeatedly, discuss, negotiate, make a proposal, listen [to their feedback or counterproposal].”
Weber’s efforts bore fruit and the rest is history – a molecule from Shionogi helped Peninsula create a $245 million exit; then a molecule from Takeda made Peninsula spinout Cerexa a $580 million exit; then a molecule from Astellas that helped Cerexa spinout Calixa (where Weber had become interim CEO) be sold in 2009 for over $400 million to Cubist.
Improving the odds
The string of exits has continued for three reasons, none of them dumb luck:
(1) Weber and Domain got results with their early efforts, undoubtedly earning the drugs’ original Japanese owners much higher returns than in traditional pharma-to-pharma licensing deals;
(2) The early results translated into repeat invitations to visit Japanese pharma companies and look in their cupboards. “Eventually,” after some exits, said Weber, “we became a well-known quantity. [Our prior deals] opened a lot of doors. We established a relationship with most of the Japanese pharma companies because we’ve done deals with them and made them want to do more deals. Now we are approached regularly.”
(3) Domain and its syndicate partners knew what to do with the drugs once it had the rights. Borrowing a model from the medical device world, where management teams are often created around assets identified by investors, Domain drew upon its reservoir of accomplished management teams and consultants – including Weber himself – during diligence, company-building and clinical development. “It’s not just a question of finding the products but also creating value in them,” he said. “We spend extensive time evaluating products and their positioning,” Weber said. “There is a great deal of [time and effort spent] developing a clinical and regulatory strategy,” which he characterized as one of Domain’s big value-adds. “Products can fail because of poorly conceived clinical and regulatory strategies.” It’s not that products never fail in Domain’s hands – sometimes they do – but the firm’s work pre- and post-closing of a deal has managed to improve the odds.
Before the window slammed shut
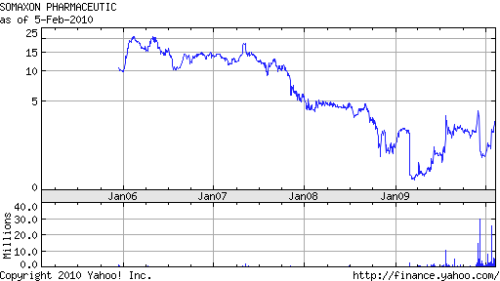
But take the deep dive into Domain’s portfolio further, all the way back to the days when the IPO was still a viable exit, and BBW found a more conventional story. Like many VC firms, Domain was – at least until 2007 – able to shed large chunks of its more speculative investments onto public-markets investors, before the risk of early-stage drug development was taken out. Yes, Domain had several IPO exits in the 2005-2007 time frame. But some of them (Novacea in the prostate cancer space; Northstar Neuroscience in medical devices) did not provide better than modest exits when their shares had to be sold at cost or the companies shut down. In this, Domain’s performance was roughly consistent with the performance of other funds in those years. The chart for Somaxon shows a typical pattern: a strong IPO and post-IPO performance followed by a disappointment in clinical development – in this case in a delay in the approval of an insomnia drug – resulting in a slide in the stock price (see Figure 2).
This type of investment – any investment that requires an IPO exit along the way – no longer fits the risk profile of what Domain is doing these days, or many other financial VC firms for that matter. The retail investor is not buying or buying only at a discount, as evidenced by the years-long near-absence of IPOs followed by Ironwood’s haircut. VCs have to expect to remain invested and involved in the companies until they either have been generating years of strong revenues – which is usually too long – or until they get acquired by pharmaceutical companies. And if the exit will be an acquisition anyway, why have the IPO at all?

All of these trends help explain why the 2010 JP Morgan healthcare conference, while it felt much more vigorous than the previous year’s shell-shocked atmosphere, continued to have as its main focus a dialogue that had become prevalent in the 2005-6-7 time frame: pharma-VC discussions. VCs are more dependent on pharma than ever for virtually every phase of their business: exits; deal flow (pharma spin-outs being one of the more “sure-thing” investment vehicles VCs invest in, although most VCs have found these in US and Europe-based pharma and it has been Domain finding them in Japan); reality checks in regard to “what pharma is buying” – what indications, what types of molecules etc.; and fund-raising (pharma companies are often limited partners in VC funds). The days when JP Morgan was a place for VCs to “look at deals” and to kibitz with entrepreneurs seem to be numbered. Many of the VC firms may not be around in a few years to share in the dialogue.
Steady as she goes
Based on the source of many of its most valuable assets, Japanese pharma, Domain can expect to continue its string of successes for years to come. “These [Japan-based pharmaceutical] companies all have R&D. They are replenishing the pipeline,” said Weber. And now, of course, Domain has the inside track.
But for the VC industry as a whole, the outlook is not so promising, at least for the next two or three years. Many VC funds have postponed raising new funds until 2010, which was probably wise given the nasty environment of 2008-2009. But now these funds really need to raise money, and their performance – which if it does not include an Eckard Weber deal or one of the few comparable high-value exits of recent years – will not make it easy.
This rough patch will likely hit Domain too as it searches for the syndication partners it needs to raise $30 million to $40 million per company. It may find itself doing big deals like this with fewer partners who each put in more; optioning rights for other geographies (e.g. Europe) earlier to raise cash for development; or selling the companies to the pharmaceutical industry, painful as it might be, earlier and for less.
Still, Domain’s and Weber’s impressive winning streak stands out against the gloomy backdrop. As long as Weber finds the pursuit of new medications more appealing than golf at Torrey Pines, there is every reason to expect it to continue.
# # #
Disclosure: Domain and the companies mentioned in this piece are not consulting clients of CBT Advisors
| Company | Location | Indication | Source of Compounds | Acquirer or IPO | Year of Acq’n or IPO | Price at Acq’n or Value* post-IPO |
| BiPar | SF Bay | Oncology | In-house | Sanofi | 2009 | ≤$500M |
| Cabrellis |
San Diego | Oncology | Dainippon Sumitomo | Pharmion | 2006 | ≤ $94M |
| Cadence | San Diego | Acute pain | In-licensed | IPO | 2006 | $500M by ‘07 |
| Calixa |
San Diego | Infectious Dis. | Astellas | Cubist | 2009 | $403M |
| Cerexa |
SF Bay | Infectious Dis. | Takeda | Forest | 2006 | ≤$580M |
| Conforma | San Diego | Oncology | In-house | Biogen Idec | 2006 | ≤$250M |
| Corthera | SF Bay | Heart failure | Connetics | Novartis | 2009 | ≤$620M |
| GeneOhm | San Diego | Diagnostics | In-house | Becton Dickinson | 2006 | $230M |
| Intralase | Irvine | Eye device | In-house | AMO (Abbott) | 2007 | $808M |
| Novacardia |
San Diego | Congestive Heart Failure | Kyowa Hakko Kogyo | Merck | 2007 | $350M |
| Nuvasive | San Diego | Orthopedics | In-house | IPO | 2004 | $500M by ‘07 |
| Orexigen |
San Diego | Obesity | In-house drug combination | IPO | 2007 | $400M by ‘08 |
| Peninsula |
SF Bay | Infectious Dis. | Shionogi | J&J | 2005 | $245M |
| SenoRx | Irvine | Onc. device | In-house | IPO | 2007 | $150M by ‘08 |
| Somaxon | San Diego | Insomnia | Reformulation | IPO | 2005 | $300M by ‘06 |
| Volcano | San Diego | Cardio device | In-house | IPO | 2007 | $900M by ‘08 |
| Vanda | Rockville, MD | Schizophrenia | Novartis | IPO | 2006 | $600M by ‘07 |
Table 1: Domain exits 2005-2009. Eckard Weber deals marked with a “W”.